
Vedanta Biosciences, Cambridge, MA
www.vedantabio.com
Vedanta Biosciences’ new VE303 drug will prevent life-threatening
C. difficile infections by boosting the body’s microbiome
Innovative microbiome project first funded by CARB-X gets funding from BARDA to progress through late development
Vedanta Biosciences, a leader in microbiome research and founded by Boston-based PureTech, has recently been awarded up to $76.9 million from the US Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response at the U.S. Department of Health and Human Services (HHS) to develop VE303 for prevention of recurrent Clostridioides difficile (C.difficile) infections in high-risk patients. The award is initially for $7.4 million, plus additional payments worth up to $69.5 million if the project progresses successfully. BARDA collaborated with the HHS Office of the Assistant Secretary for Health on the project.
The VE303 project is the first CARB-X-funded therapeutic to receive follow-on BARDA support for late-stage development and is the first live bio-therapeutic product (LBP) project in BARDA’s Antibacterials portfolio. The support from CARB-X enabled Vedanta to advance VE303 rapidly through a Phase 1 clinical trial, demonstrate favorable safety and pharmacokinetics/pharmacodynamics, and select dosing regimens for the Phase 2 study. BARDA funding will support completion of an ongoing Phase 2 clinical trial and further clinical development of VE303 toward eventual market authorization.
The microbiome – the trillions of cells that move through the human digestive system – and the crucial role it plays in the immune system, human health and other physiological processes – is one of the most exciting new frontiers in medicine today. A growing body of scientific research over the last 15 years has uncovered the microbiome’s role in fighting serious infections, including infections caused by antibiotic-resistant and hard-to-treat bacteria.
“I don’t think there’s any other emerging field of medicine today that holds as much promise for the future of medicine as the microbiome,” says Dr. Bernat Olle, co-founder and CEO of Vedanta, which is based in Cambridge, MA, USA.
VE303 is attracting attention for its exciting potential as a preventative therapy for serious recurrent C. difficile infections, which cause life-threatening diarrhea and colitis (an inflammation of the colon), mostly in people who have had recent medical care and received antibiotics. In the United States, C. difficile causes up to quarter of a million infections each year; an estimated 20 percent of these patients suffer recurring infections, and the infection is responsible for at least 12,800 deaths annually.
This bacterial infection is often associated with antibiotic use, particularly prolonged use of certain classes of broad-spectrum antibiotics. Antibiotic treatment can deplete the human body’s natural, protective, gastrointestinal bacteria, allowing C. difficile to colonize and overgrow in the intestines. In addition, C. difficile infections are a particular concern with large-scale use of antibiotics to treat secondary infections during a pandemic, such as those caused by H1N1 influenza or SARS-CoV-2, and in treating infections caused by biothreat agents, such as anthrax or tularemia, that require 60 days or more of antibiotic therapy.
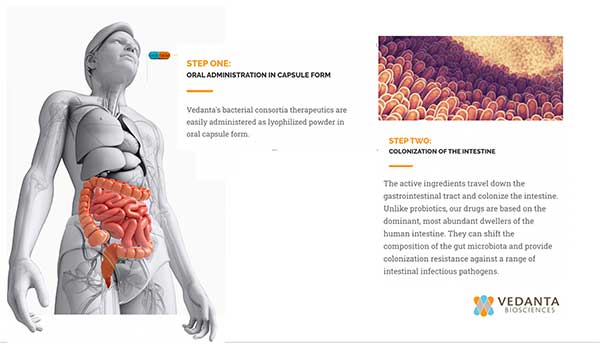
Vedanta’s microbiome restoration approach is an alternative, as well as an adjunct, to antibiotics and if successful, this restoration could lessen the need for use of antibiotics or for fecal transplants which requires identifying and recruiting human donors. VE303 is a rationally defined, orally-administered live biotherapeutic product (LBP) consisting of eight bacterial strains designed to colonize and restore gut microbiota to prevent recurrent C. difficile infections. The way the treatment works is by repopulating the patient’s gut with healthy bacteria that can fight against pathogenic bacteria.
Vedanta’s ongoing phase 2 study (NCT03788434) is a multi-center, randomized, double-blind, placebo-controlled trial designed to evaluate the safety and efficacy of VE303 in patients with a recent diagnosis of C. difficile infection (CDI), who have completed a course of antibiotics but remain at risk for recurrence.
Vedanta microbiome project VE707 targets MDRO
CARB-X is currently supporting another exciting Vedanta microbiome program, VE707, targeting other serious pathogens which result in potentially devastating outcomes for patients. VE707 consists of a defined consortium of commensal bacteria, different to that of VE303, which is being developed to eliminate intestinal carriage of carbapenem-resistant Enterobacteriaceae (CRE), extended-spectrum beta lactamase producers (ESBL), and vancomycin-resistant Enterococci (VRE) to restore a healthy microbiota as well as prevent infection and colonization recurrence.
CRE, ESBL, and VRE infections are some of the most common hospital-acquired infections and are estimated to affect over 500,000 intensive care unit, dialysis, solid organ transplant, and hematopoietic stem cell transplant patients each year in the US and Europe. Infections with these organisms can result in life-threatening treatment delays or death, and result in approximately $2 billion in healthcare-associated costs due to patient isolation practices alone. According to the Centers for Disease Control and Prevention (CDC), 15,600 Americans died in 2017 alone from these infections.
Dr. Olle says: “If we could get rid of intestinal carriage of these MDROs in high risk patients, we could not only prevent infections, but also curb the transmission of these organisms and enable physicians to avoid using antibiotics that select for ever-more resistant bacterial strains.”
VE707 is also designed to be administered orally. Like VE303, it is produced from pure, clonal bacterial cell banks, which yield a product of uniform composition and free of any pathogenic strains, bypassing the need to rely on fecal donor material with inconsistent composition.
Bernat Olle
Originally from Catalonia, Olle came to the US in 2002 to study chemical engineering at MIT, where he focused on the emerging science of using live bacteria to produce drugs. In 2007, after earning both an MIT doctorate and an MBA from the Sloan School, he joined PureTech Health. Later In 2010, PureTech backed him in launching Vedanta with five cofounders. In addition to CARB-X and BARDA funding, Vedanta has also received funding from the Bill & Melinda Gates Foundation to support preclinical research aimed at developing a gut bacteria–derived drug that would prevent child malnutrition in the developing world and scaling manufacturing of defined consortia drugs.

For more information, https://www.vedantabio.com/platform
Published on November 20, 2020


