
Peptilogics, Pittsburgh, USA
https://peptilogics.com
Breaching biofilms to kill bacteria
PLG0206 peptide aims to prevent life-threatening infections in patients undergoing knee replacements
By Robin Berghaus
As Peptilogics completes its second clinical study, the company will present interim data at a scientific meeting on August 4, 2023 for PLG0206, its first antibacterial drug candidate for patients undergoing knee replacements.
Over time, arthritis, trauma, obesity, and normal wear-and-tear can break down cartilage which serves as a cushion between bones. For those who lose this protection in their knee joints, the simple act of walking can cause debilitating pain.
Every year, more than 1 million people in the US undergo knee replacements to alleviate their pain. Although the procedure is common, patients face serious risks. These include life-threatening prosthetic joint infections (PJIs), which share the same survival odds as breast cancer. Nearly a quarter of patients treated for PJIs die after five years.
PJIs are often difficult to treat. The bacteria that cause these infections produce a shield called biofilm, a sticky substance which accumulates on prosthetic joints. Many antibiotics are unable to penetrate biofilms, rendering them ineffective. Patients with PJIs must often undergo additional surgeries to have their infected joints replaced and treated, which can result in months of rehabilitation and limb loss.
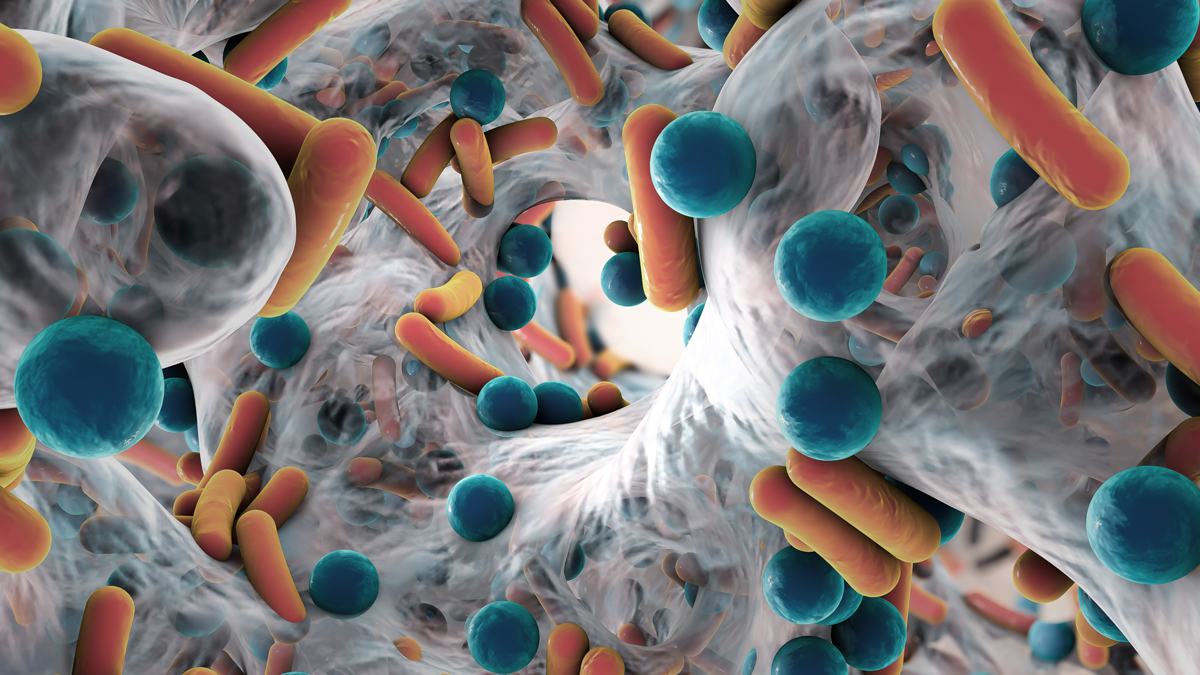
Some bacteria produce a shield, called biofilm, which comprises biopolymers including DNA, RNA, and polysaccharides. Many antibiotics are unable to penetrate biofilms, rendering them ineffective. Photo Adobe Stock
PLG0206 breaks through biofilms to kill bacteria
Peptilogics is addressing this unmet medical need with PLG0206, a small protein–called a peptide–that can penetrate biofilms and target a range of bacteria, including the most dangerous bacterial species that cause PJIs.
In Peptilogics’ clinical study, PLG0206 was administered as a topical solution during knee replacement surgery to help treat infections by killing bacteria that may be present around newly implanted prosthetic joints.
A topical application enables bacteria to be targeted directly, while also reducing the risk of toxicity. Since knee joints have limited access to blood supply, the amount of PLG0206 to enter patients’ bloodstreams is likely to be low, and an earlier human study conducted by Peptilogics demonstrated no significant safety risks.
“PLG0206 is performing as well as we ever would have hoped,” says Jonathan Steckbeck, PhD, MBA, founder and CEO of Peptilogics. “The biggest challenge is just getting other people to believe it. There’s a lot of baggage associated with this class.”
Steckbeck and his team aim to show that this class of peptides–which have a reputation for being toxic and short-lived–can be designed as safe, stable and economic solutions to treat bacterial infections.
The birth of Peptilogics
Steckbeck’s early research was focused on HIV and the human immune response to the virus. In 2005, his father-in-law, Walter Craigo, had difficulty breathing and checked into the hospital–the aftermath changed the course of Steckbeck’s career.
“While they were trying to intubate him, they nicked his trachea. That turned bad immediately,” says Steckbeck. The nick led to a bloodstream infection, and Craigo died seven days later.
“You know academically that infections and drug resistance exist. But that was a very clear crystallization that this stuff actually matters. The deeper you get, you begin to understand how dire it is and will become if we don’t do something about it,” says Steckbeck.
With effective HIV drugs already on the market, and a vaccine path that seemed years away, Steckbeck left HIV research behind. In 2006, he enrolled in a PhD program at the University of Pittsburgh School of Medicine to discover and commercialize new antibacterial products that could help save the lives of patients like Craigo.
Over the next five years, Steckbeck engineered a promising antibacterial peptide. After completing his PhD program, he founded Peptilogics in 2013.
The discovery and potential of peptides
Peptides have been studied and developed as therapies for decades. Among the most famous is insulin, a peptide hormone that controls blood sugar levels for patients with diabetes. One of the greatest achievements in the history of drug discovery, insulin has saved countless lives since it was first given to patients over 100 years ago.
But it wasn’t until the 1980s that antibacterial peptides were described. Researchers, led by Michael Zasloff, observed African clawed frogs that live in dirty water, and wondered why the frogs did not develop infections when they were cut. Zasloff and colleagues discovered a class of peptides–later identified as magainins–which reside on the frogs’ skin and kill bacteria. “These peptide molecules were found to be widely distributed in nature, from yeast all the way to humans,” says Steckbeck. “They’re part of an innate defense response.”
Over the years, as researchers characterized antibacterial peptides, they observed common sequence and chemical features. Steckbeck’s graduate lab noticed some of these peptides inside HIV. “As peptides that can kill bacteria, they had no business being inside a virus,” said Steckbeck. “But we thought they could be interesting drug candidates.”
Steckbeck’s lab began testing how they could manipulate the structure of these peptides to affect antibacterial activity–among these was the precursor to PLG0206.
Then an industry-wide breakthrough happened.
Roche and Trimeris were developing an HIV fusion inhibitor. “They were going to need a lot of raw materials, including amino acids, so they rebuilt the entire global supply chain,” says Steckbeck. “They increased the maximum scale from grams to metric tons. Prior to that, you could not make peptides at drug scale.”
PLG0206 surmounts common obstacles
Peptide developers face several challenges. Among them is how to engineer peptides that can kill bacteria, while avoiding unwanted toxicity.
Colistin is a last-resort antibacterial peptide known to be toxic. While the mechanism of toxicity is not fully understood, colistin is part of a peptide class called polymyxins which have a circular molecular structure. As colistin hangs around, it is reabsorbed and concentrates at high levels in kidneys, where it can damage patients’ own cells and cause kidney failure. In contrast, PLG0206 was constructed as a short linear peptide that Peptilogics anticipates will be broken down quickly, like other common small proteins.
While that simple structure may reduce toxicity, it led Peptilogics to overcome another challenge: instability. So PLG0206 was designed to have a high affinity for bacteria.
As for breaching biofilms, Steckbeck says “they were once believed to be impenetrable masses, but that’s not true. Biofilm comprises biopolymers including DNA, RNA, and polysaccharides which are structured communities with waste channels. So stuff can get in.”
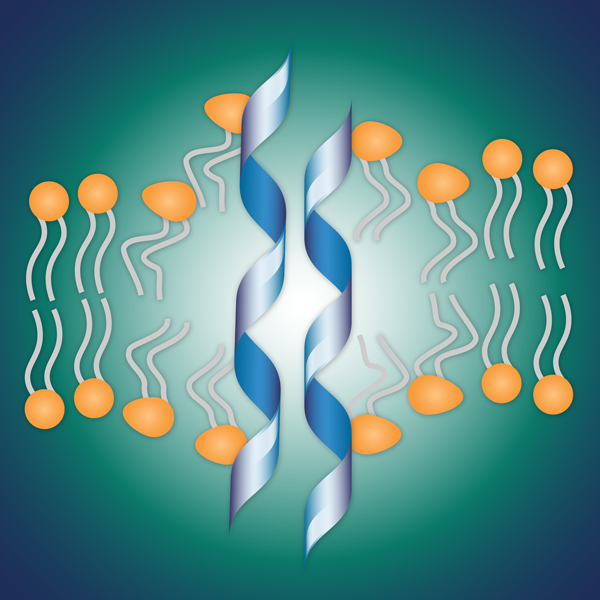
After PLG0206 penetrates biofilm, the peptide directly kills bacteria by disrupting the cell membranes.
Steckbeck believes that PLG0206 is drawn into biofilms because while the peptide is positively-charged, biofilms are negatively-charged, and opposites attract. “But if there’s one place that PLG0206 wants to be, it’s embedded in a bacterial membrane. The biofilm acts like a magnet that attracts PLG0206 to its natural home.”
Once inside, PLG0206 disrupts the cell membrane, which directly kills the bacteria. Unlike most antibiotics, PLG0206 is not a growth inhibitor. “The reason that matters is that if bacteria aren’t growing, PLG0206 can still kill them,” says Steckbeck.
Within biofilms, there are bacteria that are actively growing to maintain the colony. The others Steckbeck calls “freeloaders,” because they remain in a viable but resting state. “Even if you bathe the biofilm for a day in a high concentration antibiotic solution that inhibits growth, the freeloaders would grow back. In contrast, with PLG0206, we showed that it can eradicate all bacteria in biofilm within minutes.”

Photo of Jonathan Steckbeck by Asia Kepka
Pivoting to treat prosthetic joint infections
Steckbeck founded Peptilogics in 2013, one year after the GAIN Act was passed. The legislation was intended to help stimulate antibiotic innovation. With new incentives, including five years of additional non-patent marketing exclusivity, it seemed like a great time to get into antibiotic development.
PLG0206 was originally designed to be used systemically as a traditional peptide antibiotic. In 2020, the company completed a clinical study in Australia with volunteers who received PLG0206 intravenously at high exposures. “We didn’t see any toxicity issues associated with peptides, and it appeared to have a good safety profile,” says Steckbeck.
But as other small antibiotic companies struggled to recover their R&D costs and began filing for bankruptcy, Peptilogics pivoted.
The company researched other indications, and quickly honed in on PJIs. They believed PLG0206 would not only effectively treat these infections, but that this indication could also provide a real business model.
“If you think antimicrobial resistance is a problem, which it is, then consider PJIs,” says Steckbeck. “For these patients, the drugs don’t even work.”
Steckbeck witnessed his friend, who had been an active Navy pilot deteriorate after his prosthetic joint became infected. He required four surgeries and 18 months of rehabilitation to clear the infection. Beyond the physical pain, Steckbeck recognized the psychological toll. “It’s similar to the fear that many cancer survivors have, always wondering whether it will come back.”
The failure rate of prosthetic joint surgeries due to infections is high, ranging from 25-50%. Each surgery costs about $100,000, and hospitals and insurance companies are often on the hook for additional costs. While the total PJI patient population is small with nearly 50,000 patients per year, annual associated hospital costs in the US are estimated to climb to $1.85 billion by 2030. “So hospitals and insurance companies have a real economic incentive to prevent these infections,” says Steckbeck.
PLG0206 clinical trials
In October 2022, Peptilogics began a clinical trial with 14 patients undergoing debridement, antibiotics, and implant retention (DAIR) for treatment of a PJI occurring after a knee replacement. To design an effective trial, the team had to overcome unique challenges.
“At our 12 study sites, the surgeon’s approach to each patient is unique,” says Nicholas Pachuda, DPM, Chief Operating Officer at Peptilogics. “We cannot standardize an operative procedure, but we can control how PLG0206 is administered. We integrated into the surgeon’s routine workflow and a Peptilogics’ clinical representative was in the operating room to answer questions, so PLG0206 was adopted without complication.”
As a fully remote operation, Peptilogics team members live in regions across the nation from Pennsylvania to Texas, which enables them to visit operating rooms on short notice.
Beyond limiting surgical variables, the team had to navigate a small patient population. Among those who undergo knee joint replacements, PJIs occur in 1-2% of the patients. Because the infection rate is rare, the FDA granted PLG0206 orphan drug status. “Peptilogics was careful selecting clinical trial sites that represented a cross-section of hospital settings, from large academic tertiary care centers to busy regional hospitals. With the right protocol and inclusion and exclusion criteria, we drove rapid enrollment without creating too much heterogeneity,” says Pachuda.
Pachuda credits the CARB-X and Peptilogics internal R&D teams, and their Surgeon Advisory Board with creating an effective clinical trial design. “Peptilogics learned from these highly experienced teams. We worked together on site selection, enrollment criteria and the comparators of the standard of care antibiotics. We are fortunate to continue to work with these teams on our clinical, regulatory, reimbursement and commercial strategies.”
CARB-X has awarded Peptilogics approximately $12 million toward the development of PLG0206. “Joint replacements are increasingly common, and infections are a serious and costly complication,” says Erin Duffy, PhD, CARB-X Chief of Research and Development. “During the period of our support, PLG0206 matured to have the performance characteristics that could represent tremendous improvement in the treatment of periprosthetic joint infections.”
In 2022, the trial safety review committee found no treatment-related adverse effects in the first patient cohort. As such, it approved a higher dose for an additional 7 patients in the second cohort who are undergoing DAIR, a surgical procedure in which infected tissues around the prosthetic knee joint are removed and treated.
Since PJIs can take months to develop, Peptilogics will follow patients for one year to assess safety and signs of infection. By late next year, they anticipate beginning a Phase 2/3 DAIR trial, which should establish clinical proof-of-concept.
Peptilogics will present interim data from the PLG0206 clinical trial on August 4, 2023 at the MusculoSkeletal Infection Society’s Annual Scientific Meeting in Salt Lake City.
Scaling a diverse pipeline using artificial intelligence
Peptilogics views PLG0206 and treating PJIs as just the beginning for its promising pipeline.
“Medicine hasn’t progressed too far from the Civil War era when it comes to orthopedic infections. When doctors can’t cure the infection, they amputate,” says Steckbeck. “We see an opportunity to use the unique characteristics of PLG0206 to help more orthopedic patients and go broader.”
PLG0206 as well as Peptilogics’ second molecule, PLG0301, are also being developed with support from the Cystic Fibrosis Foundation for their antibacterial and anti-inflammatory properties. Patients with cystic fibrosis are prone to respiratory infections, as their lungs accumulate a thick mucus, which becomes a breeding ground for bacteria. Repeated bouts of infections and inflammation can deteriorate the lung tissue and lead to respiratory failure.
While PLG0206 was designed in a traditional, manual process to increase antibacterial activity and decrease toxicity risks, the company is now using artificial intelligence (AI) to speed up the discovery process of new peptides. AI is also viewed as a boon to their company that operates remotely and outside the bounds of a traditional laboratory.
“If we could understand how all drug-like properties relate to peptide sequence and structure, we could design new molecules computationally,” says Steckbeck. “We still have to make them and test them in the lab, but we can focus on the ones that we believe have a high probability of working.”
Jonathan Steckbeck
CEO and Founder

Photo of Jonathan Steckbeck by Asia Kepka
Jonathan Steckbeck is the founder and CEO of Peptilogics, a clinical stage biotechnology platform company pioneering the development of best-in-class novel peptide therapeutics. Under his leadership, Peptilogics has developed a computational drug discovery platform to speed the design and development of novel peptide drugs across a range of therapeutic targets. The company is now rapidly advancing a pipeline of anti-infective therapies in focused indications and expanding the application of its platform to new disease areas. Steckbeck has a PhD in Biochemistry and Molecular Genetics and an MBA from the University of Pittsburgh.
Nicholas Pachuda
Chief Operations Officer

Photo of Nicholas Pachuda courtesy of Peptilogics
Nicholas Pachuda serves as the Chief Operating Officer of Peptilogics and has over 20 years of industry experience in addition to working for 10 years as a surgeon. He has been focused on orthopedic infections his entire career, both in the clinic, research, new venture creation, and investments. Prior to joining Peptilogics, he led External Innovation for the orthopedics business of Johnson & Johnson and created multiple investments and partnerships in the anti-infective space. He has been part of two successful startup exits and led global teams for Johnson & Johnson and Synthes. Pachuda earned his undergraduate degree from the University of Pittsburgh, and his DPM from the Pennsylvania College of Podiatric Medicine. He did his surgical training at the University of Medicine and Dentistry of New Jersey. He also completed an Orthopedic Trauma Fellowship in Germany.
For more information, peptilogics.com
Published on August 3, 2023


