
Pattern Bioscience, Austin, Texas, USA
https://pattern.bio
Using Patterns to Decode a Sea of Bacteria
First-in-Kind Diagnostic Identifies Bacterial Pneumonia and Provides Definitive Antibiotic Susceptibility Results in 4 Hours
By Robin Berghaus
Despite developing major advances in diagnostic technology over his decade-long career, electrical engineer Nick Arab was frustrated. Accurately diagnosing a life-threatening bacterial infection took too long, putting patients’ lives at risk.
Arab saw this acutely with lower respiratory tract infections, which affect the airways from the trachea to the lungs, and include bronchitis, influenza, tuberculosis and pneumonia. These are the world’s most deadly communicable diseases, killing approximately 2.6M people every year.
Treating critically ill pneumonia patients quickly is key to their survival. Patients with pneumonia struggle to breathe as their lungs fill with pus and fluid, and they can require hospitalization and intensive care treatment when their bloodstream oxygen levels drop.
The need for rapid and definitive results inspired Arab to co-found Pattern Bioscience and create the Pneumonia Action Panel. Its debut diagnostic identifies bacterial pneumonia and provides definitive results for effective treatments in 4 hours. No other diagnostic for bacterial pneumonia with this speed and precision is available on the market.
How Pattern’s Technology Delivers Rapid, Definitive Results
Pneumonia infections caused by bacteria are treated with antibiotics. Common methods used to identify effective antibiotics require growing bacteria in culture, which can take days to return results. Meanwhile, doctors typically prescribe broad-spectrum antibiotics as a best guess until tests results arrive.
Pattern’s technology bypasses the need to grow bacteria in culture by using single-cell analysis combined with machine learning. By concentrating the sample signal into picoliter-scale droplets about the width of a human hair, Pattern curtails testing from days to hours.
While current antibiotics can effectively cure most forms of bacterial pneumonia, resistant strains are putting routine drugs to the test. Pattern’s Pneumonia Action Panel tests bacteria in the presence of antibiotics to provide phenotypic antibiotic susceptibility results. The term phenotypic refers to a visible or behavioral trait, which Pattern’s test detects by observing how the metabolism of each bacterial cell changes when the bacteria are exposed to antibiotics. With rapid and definitive results, Pattern aims to slow resistance by breaking the cycle of antibiotic overuse. A focused treatment could also spare patients the risk of toxicity and other side effects associated with some broad-spectrum antibiotics.
Pattern designed a scalable and compact modular system–approximately two feet wide–to comfortably fit on a lab bench. Each testing module accepts Pattern’s cartridges which are preconfigured with dried reagents and antibiotics. When cartridges are loaded, the samples are partitioned into picoliter-scale droplets that capture individual bacterial cells. These cells interact with fluorescent molecules to produce fluorescence-over-time waveform patterns that represent the metabolic activity unique to each bacterial species. Pattern’s artificial intelligence engine interprets those waveforms for precise pathogen identification. The same process is repeated in wells with antibiotics and in control zones without antibiotics. The waveform patterns may or may not change. These results indicate whether the bacteria are susceptible to the panel of antibiotics to help guide treatment decisions.
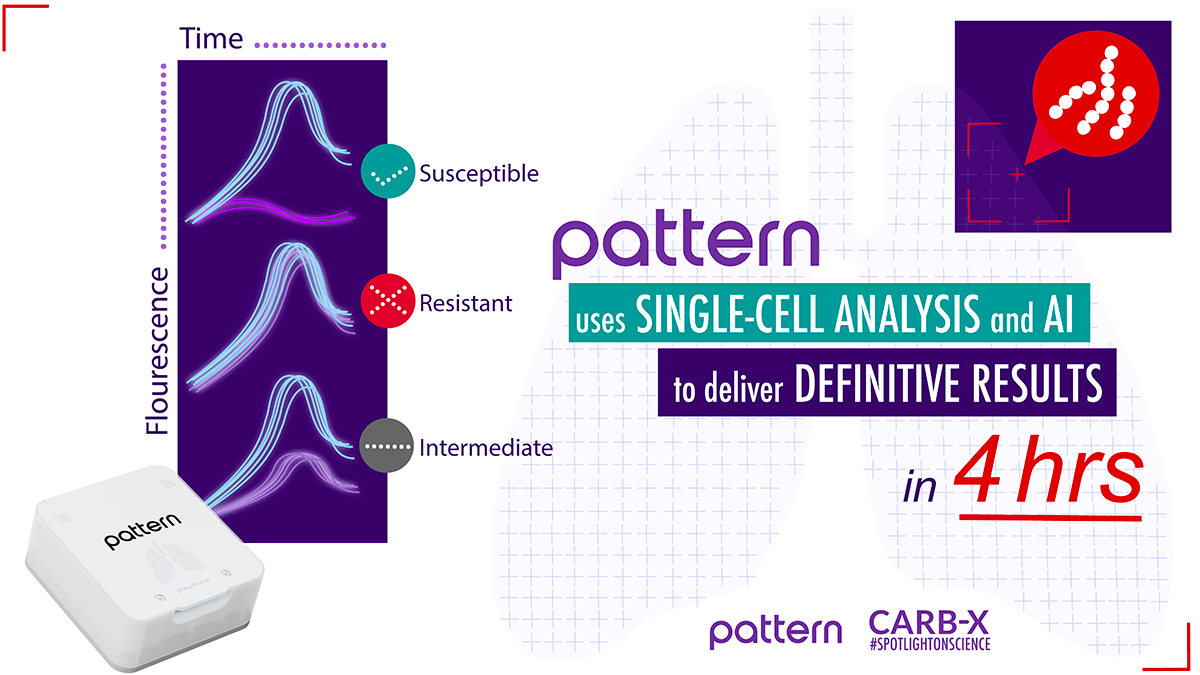
Pattern’s technology uses single-cell analysis and artificial intelligence to identify pathogens and provide antibiotic susceptibility results in 4 hours.
Within a Sea of Bacteria, Lung Samples Pose Unique Challenges
Pattern is an outlier as it develops such an innovative platform for the rapid detection of lower respiratory tract infections and antibiotic susceptibility results. Few, if any, competitors exist.
Common methods for diagnosing lower respiratory tract infections are complex. The Bronchoalveolar Lavage (BAL) method requires a pulmonologist, guided by an x-ray, to pass a bronchoscope through a patient’s mouth or nose into an airway of the lungs. A small amount of fluid is expelled into the lungs and collected, resulting in a BAL sample. If the clinician misses the location where the infection is present, the sample may not contain the bacteria, which could lead to an incorrect diagnosis.
Because a diagnosis is as good as the sample, most companies have shied away from products which rely on BAL fluid and other lung samples. Instead, companies have built their technology around less risky samples, like blood, which is easier to collect and analyze.
Arab compares diagnostics that analyze blood to COVID tests. “Because COVID shouldn’t be there, it’s a ‘yes or no’ test. And since blood is sterile, presence or absence of bacteria means something.” That’s not the case for lung samples which are easily contaminated and can contain a multitude of bacteria. The trick is trying to figure out within the sea of bacteria, which ones are causing the infection, if any.
Pattern’s test helps to overcome this hurdle by accepting polymicrobial samples, which contain multiple varieties of bacteria, and identifying each type of bacteria simultaneously and quantitatively. This helps clinicians discriminate between harmless and disease-causing organisms.
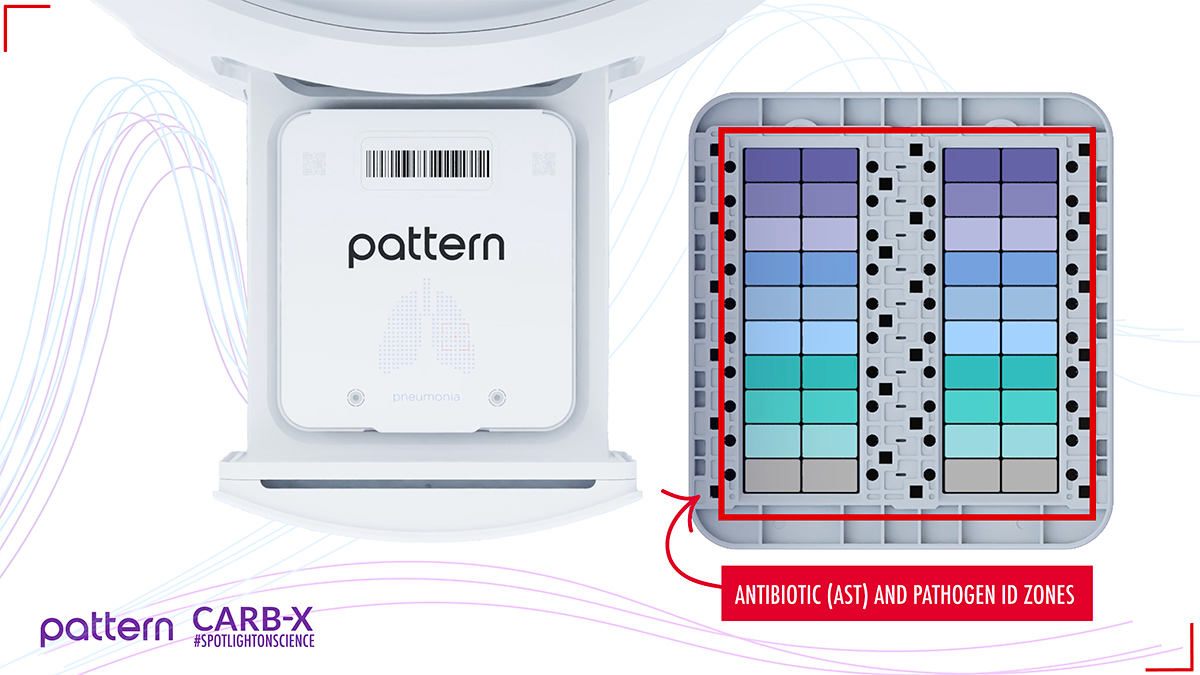
Within Pattern’s Pneumonia Action Panel cartridge there are zones for pathogen identification and antibiotic susceptibility testing.
Pattern’s technology aims to fill a critical void. “There are no rapid phenotypic diagnostics available on the market today to detect lower respiratory tract infections caused by bacteria,” says Erin Duffy, Ph.D., R&D Chief of CARB-X. Dr. Duffy explains that CARB-X is investing heavily in a portfolio of products that target lower respiratory tract infections, because they are the leading cause of deaths due to antimicrobial-resistant bacteria. “We desperately need these technologies to help save lives and curb resistance,” says Dr. Duffy.
The Birth of Pattern
After graduating University of Texas in 2002, Arab desired to stay near friends and family. Austin is not a biotech hub. But the one biotech company nearby that Arab knew about, Luminex, had an opening in its software department. Determined to use his engineering skills to help improve human health, he took the job to get his foot in the door. “I was actually doing sonar development in land-locked Austin, which is definitely not biotech,” says Arab. Eventually, he made his way to Luminex’s hardware division, and later ran its Advanced Technology Group. There, Arab learned about biology and chemistry, and how to approach health challenges from an engineer’s perspective.
But it was at a 2014 conference in Lisbon where Arab grasped the gravity of antimicrobial resistance and the technical challenges. That talk would change the course of his career.
Arab realized the critical role diagnostics play to help slow resistance. “It’s a rigged game. Bacteria are always evolving, and it takes years to bring a new drug to market. We’ll never get ahead of the problem if we’re just plugging holes with new antibiotics,” says Arab. “We need to start treating infections better to reduce the chances that resistance evolves in the first place.”
Arab returned home motivated to help. He mapped out the challenges, and where common PCR-based molecular diagnostics fell short. These tests require knowledge of genetic sequences that cause resistance. But as bacteria evolve, their DNA changes too. Molecular diagnostics are never 100% accurate, because the databases of sequences must be updated as bacteria evolve new resistance mechanisms. “So even if you could solve all the challenges associated with sequencing,” says Arab, “they may never be as good as a phenotypic diagnostic,” which directly tests antibiotics in the presence of bacteria.

Photo of Nick Arab courtesy of Pattern
Once Arab committed to solve the problem using a phenotypic approach, he knew that he would have to build it himself, since Luminex was focused on other technology programs.
When Arab co-founded Pattern with Ross Johnson and Bryan Gammon in 2016, he envisioned a test that could run directly from a patient’s sample–one that would separate single bacterial cells to avoid confusing the antibiotic response. “That’s the magic of the petri dish. It isolates cells on the surface,” says Arab. But antibiotic susceptibility testing which requires growing bacteria in culture and tests a handful of colonies leaves room for error. It is a massive under-sampling compared to Pattern’s technology which analyzes thousands of cells. “When you’re looking at resistance, you may miss the fact that some of the cells in the infection may have been resistant but are not shown in the ones that you plucked from the culture,” says Arab.
Since its inception, Pattern has made major strides. In December 2021, its rapid Pneumonia Action Panel received Breakthrough Device Designation from the US Food and Drug Administration (FDA). The device will receive priority review as the company aims to begin clinical trials in 2023.
Arab attributes the speed with which his small team advanced its technologies to their shared experience at Luminex. “We developed and cleared a lot of complicated platforms over 10 years. At other places, you may do one of these every five to 10 years.”
The Scalable Platform Could Detect a Range of Pathogens
While Pattern’s initial test will identify hospital-acquired pneumonia, the company plans to engineer additional cartridges to diagnose community-acquired pneumonia, bloodstream infections, urinary tract infections, and more. Pattern’s adaptable platform and customizable microchips could eventually be engineered to identify a range of pathogens, and to process diverse samples, from blood to urine and vaginal swabs.
CARB-X has contributed approximately $18.7 million toward the development of Pattern’s technology and its Pneumonia Action Panel, enabling Pattern to move from technical feasibility into product development. “Diagnostics is one of the hardest things to fund on the planet,” says Arab. “Without CARB-X, it would have been difficult to start a company from scratch and get a product to market.”
Beyond the financial support, Arab appreciates CARB-X’s in-house R&D team who empathized with Pattern as it entered uncharted territory. Arab describes the obstacles they faced from figuring out how to disperse reagents into small droplets, to maintaining an environment that keeps cells alive, and designing powerful algorithms that recognize unique patterns generated by cells. “This has never been done before. As things have changed, CARB-X has changed with us. They didn’t stick to something they thought we could do two years ago, which no longer made sense as the product came to life,” says Arab.
Since receiving FDA Breakthrough Device Designation, Pattern has quickly scaled up in anticipation of moving into verification and validation stage. They now have a team of 60 scientists, engineers, and other specialists, including 20 new hires so far this year. In March 2022, Pattern recruited Dr. Cary-Ann Burnham, who served as Medical Director of Clinical Microbiology at Barnes-Jewish Hospital. As Chief Clinical Officer, Dr. Burnham will oversee the clinical and regulatory strategy as Pattern takes its device through clinical trials.
“Pattern took a risk by focusing on this challenge for their debut diagnostic, and that risk is paying off,” says Dr. Duffy. “We look forward to continuing to support their product development efforts as they prepare to enter the clinic, and to all they might bring to the antimicrobial resistance market in years to come.”
Nick Arab
Co-founder and Chief Executive Officer

Photo of Nick Arab courtesy of Pattern
Before co-founding Pattern, Nick held multiple leadership positions at Luminex for more than a decade. He most recently served as senior director of the Advanced Technology Group, and won Luminex’s Top Inventor award twice. Nick is the inventor on 39 granted patents–an additional 37 are pending. He obtained his Bachelor of Science in electrical engineering from University of Texas at Austin.
For more information, pattern.bio
Published on September 27, 2022


