Breaking down the 2022-2023 funding rounds
CARB-X received applications from around the world on three themes:
![]()
Oral therapeutics
Oral therapeutics are essential to treat infections globally.
- 1/3 of the oral antibiotics on the WHO Essential Medicines List are marked as “Watch” due to a risk of resistance.
- Of the 15 new antibiotics approved by the FDA in the last decade, only 1/3 have an oral option.
- Of the 61 therapeutic programs supported by CARB-X since inception, 5 pursued an oral option, and only 2 are progressing.
![]()
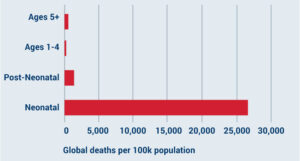
Vaccines for neonatal sepsis
Neonates are 69 times more likely to die from sepsis than children in the 5+ age bracket.
Infections that cause neonatal sepsis are particularly burdensome in low- and middle-income countries (LMICs).
CARB-X requires neonatal sepsis vaccines to target top causative agents in LMICs where commercial indications would also be possible for high-income countries (HICs).
![]()
Gonorrhea products
Ceftriaxone is the only antibiotic left that can effectively treat drug-resistant strains of gonorrhea.
Here’s what we learned:
![]()
Applicant demographics
Applicants by organization size:
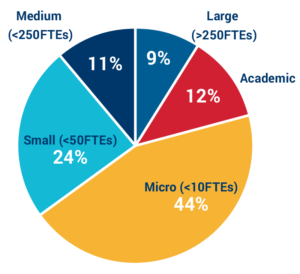
FTEs = Full-time employees
80% of applicants come from micro to small companies and academic institutions. Small teams continue to lead R&D in the antimicrobial resistance space, which contributes to the fragility of the ecosystem.
Furthermore, many of the medium and large sized groups work on multiple therapeutic areas, so the antibacterial programs typically comprise only a small portion of the workforce.
Breaking down the omnibus solicitation
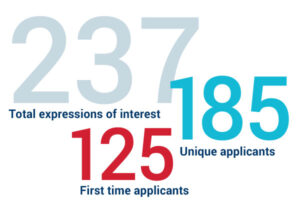 CARB-X received 237 expressions of interest. Among them, 185 were unique applicants, and 125 applied to CARB-X for the first time.
CARB-X received 237 expressions of interest. Among them, 185 were unique applicants, and 125 applied to CARB-X for the first time.
Here’s how the applications break down by theme and stage:

Tx = Oral therapeutics
Vx = Vaccines for neonatal sepsis
Dx = Rapid diagnostics for gonorrhea
FIH = First-in-human
PC = Pre-clinical
LO = Lead optimization
HtL = Hit-to-lead
Alpha Dev = Alpha Development
Trends
- Rapid diagnostics expanded during the COVID-19 pandemic.
Developers built rapid tests to detect the SARS-CoV-2 virus. This resulted in a significant increase in the number of diagnostic products in use by customers, near point-of-care diagnostics, and fully established commercial and reporting infrastructures. As the pandemic winds down, product developers are poised to capitalize on their investments by embracing new sample types and pathogens, including N. gonorrhoeae. - More than half of the therapeutics applicants are in the hit-to-lead stage.
This is consistent with the dearth of oral therapeutics in the clinical and preclinical pipelines, and the barriers that product developers face as they attempt to advance oral therapeutics to patients. CARB-X aims to help these programs advance to the clinical stage and replenish the pipeline with new oral therapeutics. - CARB-X received 35 expressions of interest from the vaccine community, who understand that there is a need for targeting pathogens of interest.
Few vaccines are in clinical development for pathogens of interest. These include:
• 1 for K. pneumoniae
• 5 for ExPEC
• 11 for S. aureus
• 0 for A. baumannii
• 1 for N. gonorrhoeae
Historically, the pathogen S. aureus has posed challenges for vaccine developers, which has resulted in a high number of failed attempts. This is why CARB-X sought new approaches to develop an effective vaccine that can target S. aureus.


