Accelerating global antibacterial innovation
Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) is a global non-profit partnership accelerating antibacterial products to address drug-resistant bacteria, a leading cause of death around the world. The CARB-X portfolio is the world’s most scientifically diverse, early development pipeline of new antibiotics, preventatives, rapid diagnostics and other products. CARB-X is the only global partnership that integrates solutions for the prevention, diagnosis and treatment of life-threatening bacterial infections, translating innovation from basic research to first-in-human clinical trials.
CARB-X is funded by a global consortium of governments and foundations. CARB-X headquarters are located at Boston University.
Our vision
Life-saving innovation keeping the world prepared for dangerous bacterial infections.
Our mission
Accelerate a diverse portfolio of innovative antibacterial products towards clinical development and regulatory approval with funding, expert support and portfolio acceleration tools.
2024 Annual Report
Accelerating Innovation to Protect Lives from Bacterial Infections
The antibiotic resistance crisis
Beginning with penicillin in 1942, antibiotics have transformed modern medicine and saved millions of lives. Antibiotic resistance occurs when bacteria evolve to render antibiotics ineffective, and represents one of the world’s greatest public health threats. 1.27 million deaths worldwide were attributed to resistant bacterial infections in 2019. Resistance is exacerbated by overuse and misuse of antibiotics. Due to poor economic incentives, many antibiotic companies have filed for bankruptcy, and large pharmaceutical companies have shuttered their anti-infective divisions. This has caused the pipeline of drug development to slow down, preventing new treatments from getting to patients. Drug-resistant infections kill more people than HIV/AIDS and malaria and any infectious disease apart from COVID-19 and tuberculosis. The best strategy for overcoming resistance is to present the bacteria with a new class of antibiotic that it has never seen before. The last new class of antibiotic approved by the FDA or EMA for use against Gram-negative bacteria – which represent the greatest threats today – was discovered in 1962. We urgently need new approaches to prevent, diagnose and treat these infections to deliver products to patients.
How CARB-X works
CARB-X fills a critical gap to help stem this crisis. Beginning with competitive funding calls to address unmet medical needs, CARB-X provides non-dilutive funding as well as scientific, regulatory and business support to product developers. CARB-X focuses both on the most serious drug-resistant bacteria and on the syndromes with the highest degrees of mortality and morbidity, globally. Diagnostics are supported from feasibility through prototype development, while therapeutics and preventatives are supported from finding potential leads for new drugs through preclinical development and into a demonstration of safety in human clinical trials.




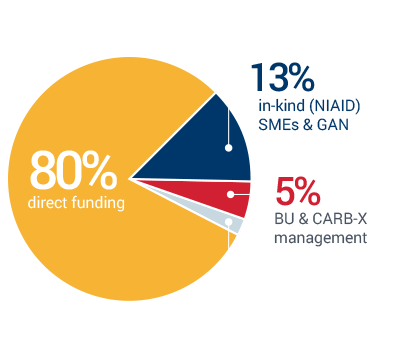 Lean and efficient CARB-X
Lean and efficient CARB-X