The backbone of modern medicine
Antibacterials are an essential part of modern medicine to prevent and treat infections.

Cancer
9.8 million people chemotherapy/yr globally. Infection is the 2nd leading cause of death for people with cancer

Organ transplants
170,000 globally per year. Patients are vulnerable to infection from surgery and suppressed immune system

Dialysis
4.35 million people with kidney disease receive dialysis or a kidney transplant, many require antibiotics

Sepsis
11 million people die of sepsis/yr. W/o effective antibiotic treatment, sepsis can lead to tissue damage, organ failure, and death
Surgery
Surgical site infections require antibiotics. Globally, 1 in 5 births are by cesarean section/yr

Chronic Diseases
Chronic conditions increase risk of infection. Many meds lower ability to fight infection. >460 million people w/diabetes
Where do antibiotics come from?
The first antibiotic was discovered in 1928 when researcher Alexander Fleming found a type of mold, Penicillium chrysogenum, had halted the growth of Staphylococcus, bacteria that can cause skin infections and pneumonia, in his petri dish. It also was found to kill other bacteria. Many existing antibiotics are derived from natural sources like soil bacteria, but other therapeutic approaches are also showing promise. Building resistance to antibiotics is a natural defense mechanism in bacteria.
Why infections are so hard to treat
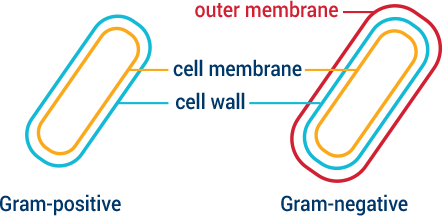
Bacteria have evolved ways to protect themselves against unwanted or toxic compounds such as antibiotics. In fact, many of the antibiotics in clinical use are chemical weapons or derivatives that bacteria created to fight other bacteria. The ways bacteria protect themselves include modifying their surfaces so invaders cannot enter, or using pumps that eject invaders if they do successfully enter. Bacteria can also change the shape of surfaces where invaders enter and evolve tools that cut or modify the invaders themselves. Gram-negative bacteria have a double membrane or wall, making them more difficult to penetrate. CARB-X targets some of the most challenging Gram-negative bacteria identified as global priorities.
Antibiotic development is not keeping pace with resistance
The global antibiotics pipeline is precariously thin. Of the 42 antibiotics in the global pipeline as of June 20181, only 11 were in development to treat superbugs on the WHO critical threat pathogen list.2 Few of the products in the pipeline are novel enough to withstand development of resistance for very long.
1 Pew Charitable Trusts, March, 2019
2 World Health Organization, “Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics”, 2017

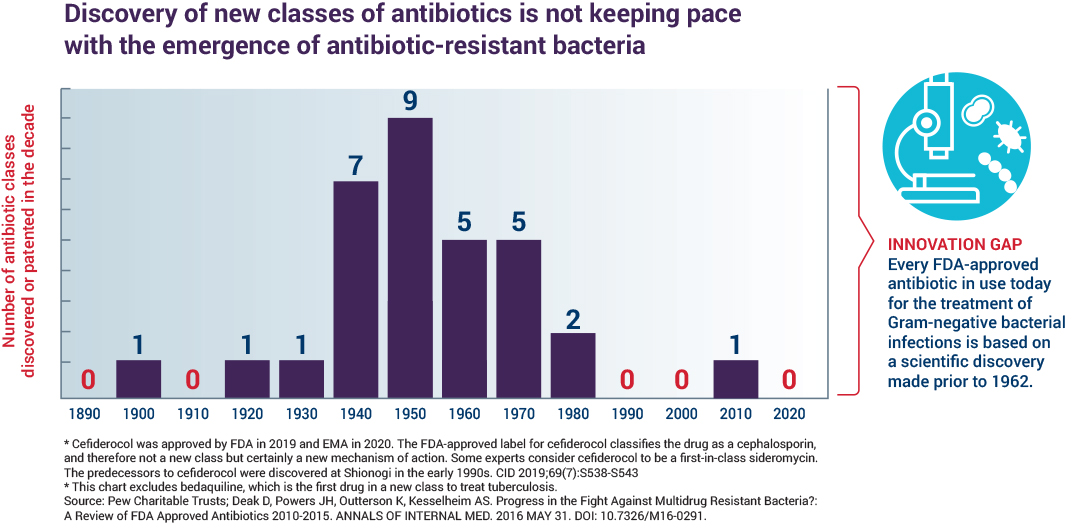
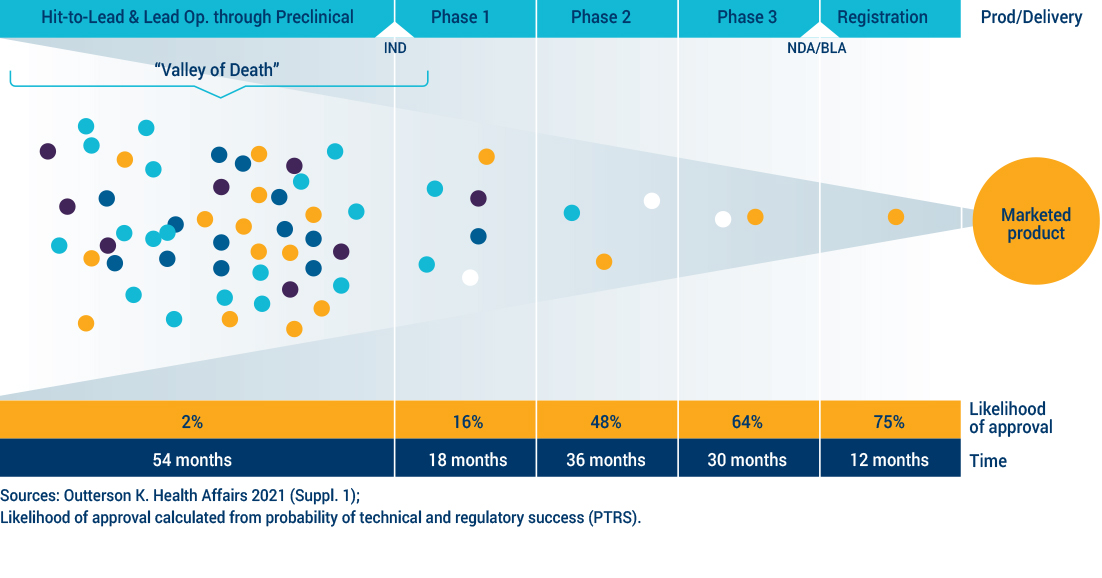
No novel classes of antibiotics have been approved for use since 1962 for a subset of bacteria, the Gram-negatives, which represent the deadliest threats today. Innovations to improve the diagnosis and prevention of drug-resistant infections have been slow, underscoring the need for new antibiotic treatments. The science is challenging, involving a balancing act of navigating to the infected site, penetrating the bacteria, remaining there, demonstrating features consistent with efficacy against the bacteria and eliminating or reducing toxicity to a human. It takes at least 10 years and hundreds of millions of dollars to develop a new drug. The economic model that once spurred a steady supply of new antibiotics no longer works. CARB-X focuses on projects in early development phases, often referred to as the “Valley of Death”, because it is in these early phases of research that many projects are abandoned because of lack of funding and support. New economic models, like CARB-X, are needed to spur further investment and innovation.